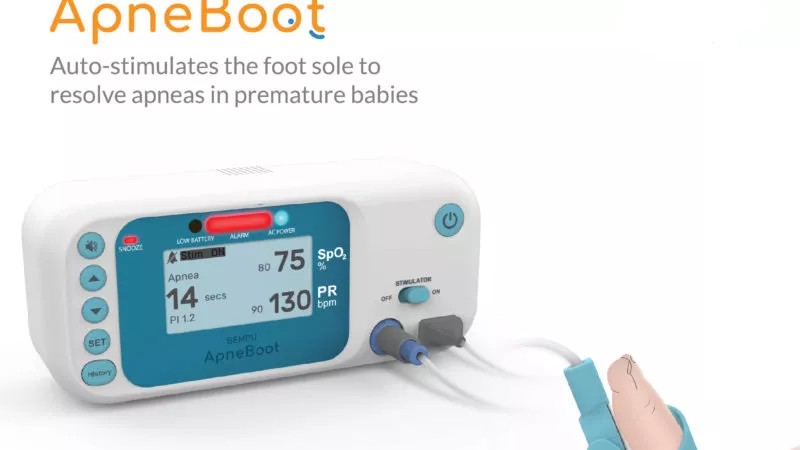
ApneBoot is a wearable device to alert for bradycardia and hypoxia in newborns.
Key Features
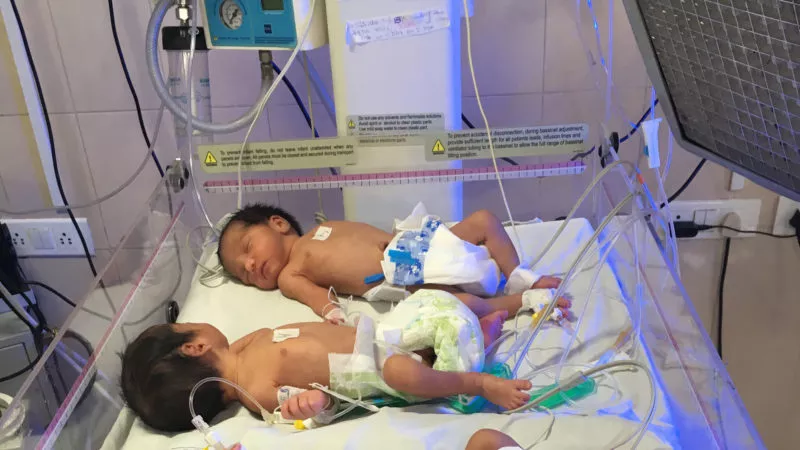
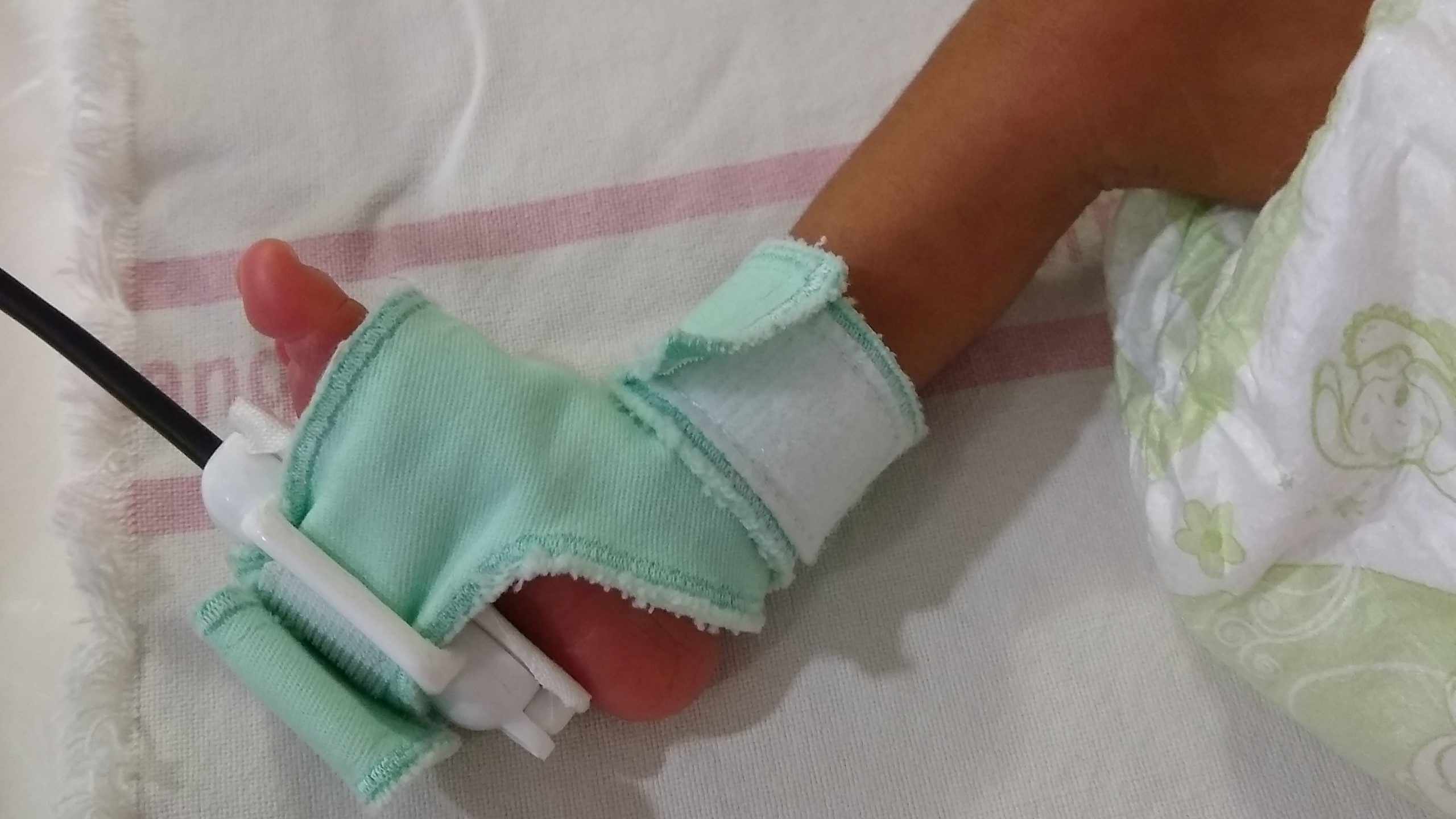
ApneBoot is a wearable gadget created by BEMPU Health to alert for bradycardia and hypoxia, two important signs of infant apnea. Additionally, ApneBoot treats protracted hypoxia events and apnea episodes in premature neonates by providing immediate tactile stimulation to the foot sole. The device fits on the infant foot in the practical shape of a baby boot and contains an integrated pulse oximeter with an alarm and auto-stimulation mechanism. The item is made to accommodate very little babies and can be worn right away after birth.
An algorithm that is already included in ApneBoot keeps track of changes in heart rate and/or oxygen saturation. If an episode occurs, ApneBoot:
● The newborn’s foot sole, which has a lot of nerve endings, vibrates. The respiration is restarted and the neurological system is stimulated.
● Emits an audiovisual warning to draw the nurse’s attention if the apnea is secondary and the treatment of the condition requires actions other than stimulation, such as assisted ventilation.
Additionally, the device contains a battery backup that allows it to operate for 12 hours without recharging, offering continuous monitoring even in situations with erratic power supplies or during transportation. It is a suitable innovation for environments with limited resources because it is lightweight, durable, reusable, and inexpensive.
Social Impact
A major risk factor for premature infants is Apnea of prematurity (AOP), which is a cessation of breathing. AOP can result in severe injury and death if not treated right away. Every year, apneas pose a risk to 15 million premature newborns worldwide and 3.3 million preterm infants in India.
Since low-resource settings lack vital sign monitoring and trained workers to respond to apneas (only 55% of Indian SNCUs have appropriate nursing staff), apneas necessitate quick action, which is impaired in these environments. Due to apnea and related hypoxia, babies in these facilities often sustain injuries or pass away.
To address this gap, we developed ApneBoot, a device to not only detect but also provide intervention to resolve AOP.
According to our theory of change, ApneBoot will prevent prolonged hypoxia and apnea in preterm babies, protecting them from harm and mortality caused by these conditions, leading to better neurodevelopmental outcomes. The device uses a high accuracy FDA, CE approved built-in pulse oximeter, has two journal articles, and has assisted more than 120 neonates by offering seamless apnea monitoring.
Future Plans
We want to improve the neonatal standard of care in NICUs by implementing ApneBoot in all low- and middle-income nations. A total of 67,600 preterm neonates will be impacted over the course of the next five years thanks to the deployment of about 5,200 devices in India and LMICs’ public and private health settings.
In response to user input, we are also creating new device versions with additional functionality.
Main Target Group
Hospitals and neonatologists caring for premature newborns in NICUs.
Main User
Premature newborns at risk of apneas.










Leave a Comment